+86-18343147735
+86-18343147735 Detection technology of A2 β-casein in dairy products
In recent years, dairy products claiming to contain A2 β-casein (abbreviated as CN) (referred to as A2 dairy products, such as "A2 Milk" and "A2 milk powder") have been priced significantly higher than comparable, standard products. The presence of A2 β-CN has become a key quality indicator for dairy products. However, no country currently has product standards for A2 dairy, posing a regulatory challenge.
Basic composition of milk
Dairy products include liquid milk, such as cow's milk, goat's milk, and camel's milk, and their products. Dairy products refer to various liquid or solid foods made primarily from milk through processes such as heating, drying, freezing, or fermentation. Because milk has become an indispensable nutritious beverage in our daily lives, the basic composition of milk ingredients will be explained here.
Milk is composed of water and dry matter. Dry matter includes fat, protein, lactose, inorganic salts, etc. The proportions of each component are usually as follows:
- Water: 85.5%-89.5% (average 87.5%);
- Fat: 2.5%-6.0% (average 3.9%);
- Protein: 2.9%-5.0% (average 3.4%);
- Lactose: 3.6%-5.5% (average 4.8%);
- Inorganic Salts: 0.6%-0.9% (average 0.8%);
Other: Trace Elements, Vitamins, Cholesterol, etc.
The total milk solids in regular milk, or the "dry matter" after the water content has been removed, represents the nutritional value of milk. Its content is usually between 10.5% and 14.5%, and can be calculated using the Fleschmann formula:
T = 0.25L+1.2F±K
Where:
- T - total milk solids (%);
- L - milk density (g/mL);
- F - fat (%);
- K - coefficient (determined based on local testing; the standard in China is 0.14).
It's worth noting that the proteins in cow and alpaca milk are complete proteins, containing all essential amino acids. Compared to breast milk, cow's milk has lower lactose and oligosaccharide content, but higher casein and mineral content. Cow's milk also has a much higher protein content than breast milk (see Figure 1).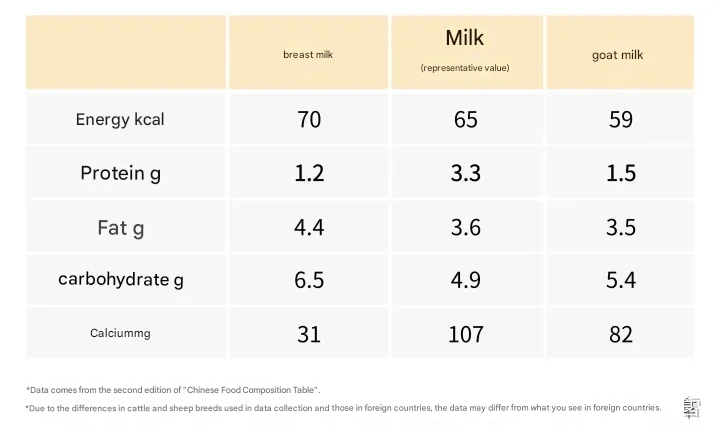
Origin and characteristics of A2-type β-CN
Milk proteins are primarily composed of whey protein and cyanocobalamin. In breast milk, cyanocobalamin accounts for approximately 50% of the protein content, while in cow's milk, it can reach 80%. GB 31638-2016 National food safety Standard - Casein defines cyanocobalamin as "a product obtained from milk and/or dairy products by acid, enzymatic, or membrane separation processes, consisting of a mixture of α, β, κ, and γ, and their isoforms." Breast milk protein is primarily composed of β-cyanocobalamin, a source of amino acids, phosphorus, and calcium for infants and young children, and forms curds in the stomach for digestion. β-cyanocobalamin is the predominant casein, accounting for approximately 30%-40% of casein. There are 12 naturally occurring variants: A1, A2, A3, B, C, D, E, F, G, H1H2, and I, with A1 and A2 being the most common. β-cyanocobalamin and its enzymatic hydrolysis products have various nutritional benefits, including promoting calcium and zinc absorption. While A1 is the predominant β-cyanocobalamin in regular cow's milk, A2 is also present in goat's and camel's milk. A 250mL glass of milk contains approximately 2g to 3g of β-CN (see Figure 2).

Research suggests that the amino acid sequence of A2β-CN is the most primitive. The A1β-CN variant currently found in cattle populations is a mutation of proline (Pro) to histidine (His) at position 67 of the A2 amino acid sequence (see Figure 3). A1β-CN can be enzymatically hydrolyzed in humans to produce 7-amino acid β-casomorphin (BCM-7), a milk-derived bioactive opioid peptide that activates endogenous opioid receptors to produce physiological responses. In contrast, A2β-CN rarely hydrolyzes to endogenous opioid peptides.
Epidemiological studies have linked BCM-7 to certain diseases, including an increased risk of type 1 diabetes, cardiac disease, sudden neonatal death, respiratory dysfunction, gastrointestinal disorders, and neurological disorders such as autism and schizophrenia. However, the extent of this association needs to be verified.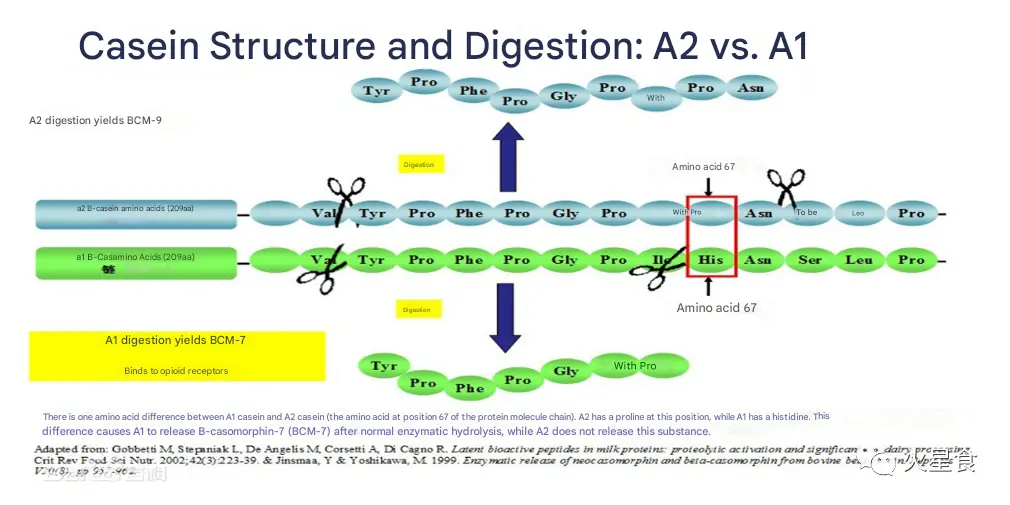
Detection Technology of A2-type β-CN in Dairy Products
At present, there are no product standards and testing method standards for A2 milk products at home and abroad. The relevant method standards and methods reported in the literature can qualitatively and quantitatively analyze β-CN.
01 Existing relevant method standards
(1) BJS 201915 Determination of casein content in milk-containing beverages and their milk raw materials. The principle is to use isoelectric precipitation to redissolve the casein in the sample solution, enzymatically hydrolyze it with trypsin, add an isotope internal standard, separate it with a C18 aqueous column, and quantify it by LC-MSMS and internal standard method. The content of four CN subtypes: αs1-CN, αs2-CN, β-CN and κ-CN can be determined.
(2) T/CNHFA 003-2022 Qualitative determination of bovine casein components in milk and dairy products. Capillary gel electrophoresis is used.
02 Analytical methods reported in the literature
It can be summarized into two categories: one is based on the research object, and analyzes the cow genotype and the final product respectively. The other is divided into qualitative analysis and quantitative determination based on the principle of the analysis method.
(1) Based on the research object
Targeting the cow genotype: The main purpose is to select and breed A2 cattle herds and manage milk sources. For example, PCR-restriction fragment polymorphism (RFLP), allele-specific PCR (AS-PCR), TaqMan probe method and rhAmp technology can be used to distinguish A1 cows, A1A2 cows and A2 cows, but it is difficult to supervise or track the cross-contamination or adulteration that may occur in the processing process and products. For example, Nguyen et al. (Food Chemistry, 2019, https://doi.org/10.1016/j.foodchem.2019.125532) used ammonium acetate precipitation of CN, reconstitution in urea buffer, separation on an Agilent Poroshell 120 EC18 column, and analysis by UHPLC-HRMS (Q-Orbitrap). This method provides molecular weight information for a range of β-CN variants with high analytical precision, enabling monitoring of the A2A2 phenotype frequency in milk produced by hybrid cows. Nguyen et al.'s results showed that the phenotypic frequency of A2A2 cows was consistently less than 1, indicating that no dairy breeds producing exclusively A2 milk were identified.
For end products, such as liquid milk and milk powder, analytical techniques primarily include HPLC, capillary electrophoresis, ELISA, and proteomics (i.e., analysis of intact proteins or characteristic peptides after enzymatic digestion using methods such as MALDI-TOF, HPLC-MS, or UHPLC-Orbitrap).
(2) Based on the principle of analytical method
Qualitative analysis includes capillary gel electrophoresis, ELISA and MALDI-TOF.
Capillary gel electrophoresis: Group standard T/CNHFA 003-2022 Qualitative detection of bovine casein components in milk and dairy products, which can qualitatively analyze bovine CN components in bovine milk, goat milk and their products, with a minimum detection limit (LOD) of 1% (ingredient mass ratio).
ELISA: Bovine A1β/A2β casein ELISA detection kit produced by Biosensis, with a detection range of 6.25ng/mL to 400ng/mL.
MALDI-TOF: Di Francesco L. et al. (Nutrients, 2018, 10, 1238; doi:10.3390/nu10091238) used this method to distinguish different types of milk. Each formula has distinct specificities. Switching from one formula to another exposes specific proteomes, thus enabling adulteration identification.
Quantitative determination is typically performed using LC or LC-MS. Sample pretreatment methods fall into three main categories:
The first involves isoelectric precipitation to extract CN, followed by washing the precipitate for detection. This method requires fewer reagents and yields pure CN, but is more complex. For example, the aforementioned method standard BJS 201915 uses isoelectric precipitation to treat samples and can determine the content of four isoforms: αs1-CN, αs2-CN, β-CN, and κ-CN. Furthermore, because this method can simultaneously measure the content of multiple CN isoforms, including β-CN, it can be combined with an A2 standard to further quantify A2 β-CN.
The second method involves protease hydrolysis to generate characteristic peptides. This method offers accurate quantification but is costly. Ehling S. et al. (Journal of AOAC INTERNATIONAL, 104(6), 2021, 1559–1566) enzymatically hydrolyzed casein in milk powder samples with trypsin, added an isotope internal standard, and separated them using a Peptide BEH C18 column. LC-MSMS and the internal standard method were used to determine the contents of β-CN and A1 β-CN. The limit of quantification (LOQ) for A1 β-CN in skim milk powder was 0.01%. In practice, this method was used to determine the content of A1 β-CN in samples made from A2 milk (ranging from 0.26% to 5.0%), and the quality of A2 dairy products was monitored accordingly.
The third method is based on the use of reagents such as urea, guanidine hydrochloride, dithiothreitol, sodium citrate, and disodium ethylenediaminetetraacetic acid to destroy casein micelles and open protein disulfide bonds, thereby denaturing the protein and detecting the protein content. This treatment method can fully dissolve proteins and unfold the spatial structure of proteins. It is simple to operate and time-consuming, but its applicable sample range is limited. For example, Lan Li et al. (Dairy Science and Technology, 2022, 45(2):18-23) used LC to determine the protein content in ultra-high temperature sterilized milk. After the sample was pre-treated with a buffer solution prepared with guanidine hydrochloride and dithiothreitol and inorganic salts, it was analyzed by LC-UV and quantified by external standard method. α-CN, β-CN, κ-CN, α-lactalbumin and β-lactoglobulin can be determined, with a detection limit of 0.01-0.02 g/100 mL. β-casein is found at a retention time of 30.0 min. There are three peaks at the β-CN of ordinary milk (type B, type A1, and type A2). The retention time of A2 type β-CN in cow milk is only A2 type β-CN at 31.5 min. This method is suitable for identifying whether sterilized milk contains A2 type β-CN. Studies have found that testing of raw milk and milk powder processed from the same raw milk revealed a 5%-10% loss in A2 β-CN content. This may be due to the Maillard reaction, which occurs when raw milk is heated during the powdering process. This glycosylation of tyrosine residues on the protein occurs, hindering trypsin cleavage, as tyrosine is the cleavage site for trypsin. However, the glycosylated CN produced by the Maillard reaction differs from the glycoproteins normally produced by the human body and can exhibit reduced and altered physiological functions. Therefore, whether the glycosylated β-CN produced by the Maillard reaction should be classified as β-CN requires further discussion.
03 Development trends and application prospects
Given the widespread application of chromatography and mass spectrometry technology, the future development of the detection of A2 β-CN in dairy products will focus on two aspects:
(1) Methodology. Based on proteomics, chromatography-mass spectrometry is mainly used to accurately identify and quantify proteins and their composition changes, and then accurately quantify A2 β-CN in dairy products. The problems that may be faced are: first, traceability, because there is currently no commercially certified β-casein or its characteristic peptide standard sample or substance (CRM); second, accuracy. If quantification is performed by characteristic peptide, based on the quantitative principle that one mole of target protein hydrolyzes one mole of characteristic peptide, alkaline trypsin cannot completely enzymatically hydrolyze β-CN, which may result in the detection value being lower than the actual value.
(2) Regulatory needs. Establishing reliable and accurate qualitative and quantitative analysis method standards and formulating product standards for corresponding dairy products such as "A2 milk" or "A2 milk powder" will not only provide technical support for dairy product quality supervision, but also provide an enforcement basis for food quality and safety supervision.
With the continuous improvement of people's requirements for dairy product quality and the increase in market demand, it is necessary to establish efficient and accurate qualitative and quantitative analysis technology for A2 β-casein in dairy products and formulate corresponding method standards and product standards. These can be used for authenticity traceability, cross-mixing or adulteration monitoring, product formula optimization and quality control of products such as "A2 milk" or "A2 milk powder", which not only protects the rights and interests of consumers, but also safeguards the economic benefits of producers.