+86-18343147735
+86-18343147735 0102030405
News

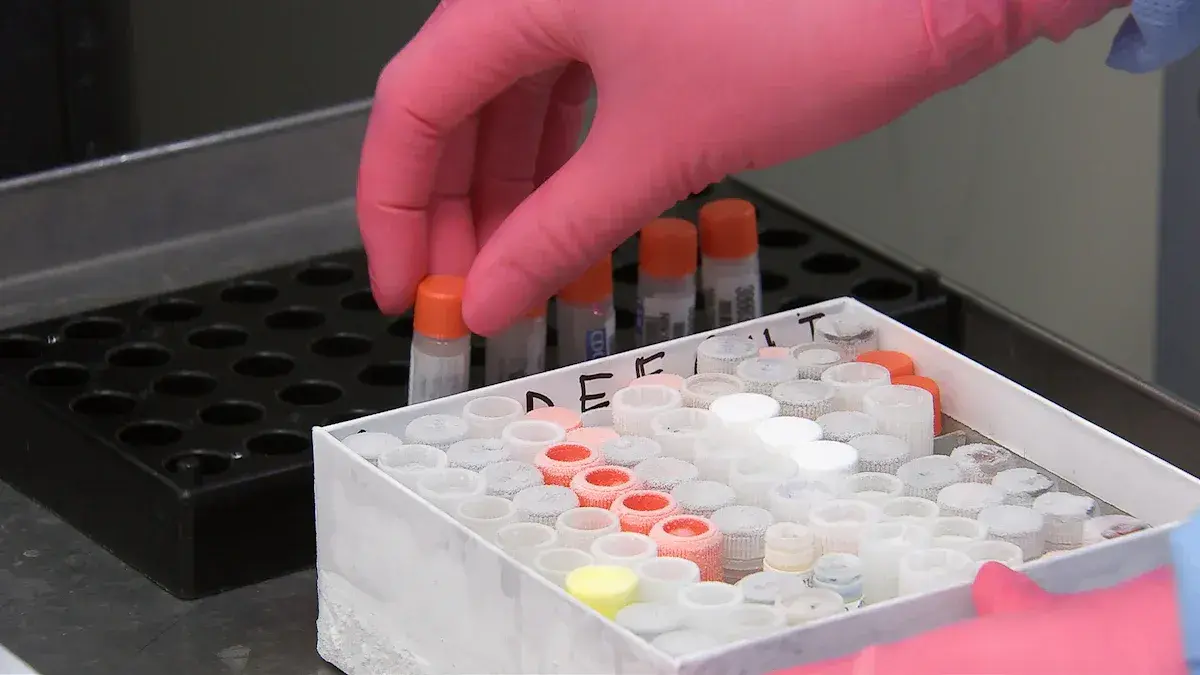
The In Vitro Diagnostic Regulation (IVDR) replaced the IVDD and entered into force on 26 May 2017 with 26 May 2022 as date of application
2025-05-16
In March 2023, the IVDR was amended as regards to transitional provisions for certain in Vitro Diagnostic (IVD) medical devices on Article 110(4) with the removal of the sell-off period to prevent ...
view detail 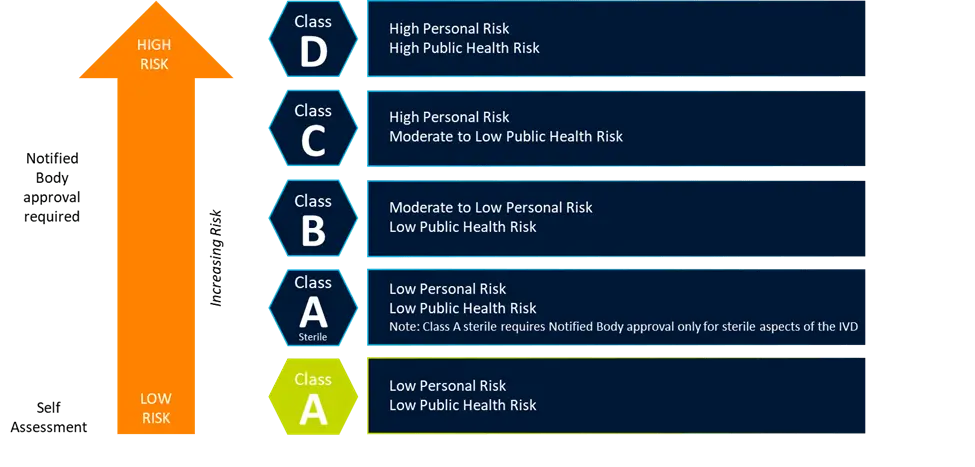
Key changes introduced by IVDR
2025-05-16
In vitro diagnostics (IVDs) have become a cornerstone of modern medicine, and rising demand for these critical medical devices has driven rapid market growth. Since 2021, hospitals and laboratories...
view detail 
Future Drivers and Challenges
2025-05-15
The In Vitro Diagnostics (IVD) market is undergoing significant transformation, driven by technological advancements, shifting market dynamics, and evolving regulatory landscapes. As we navigate th...
view detail 
The Shift from IVDD to IVDR—and the Challenges
2025-05-08
The shift from IVDD to IVDR represents a major change in how the IVD industry is regulated. While the EU Commission maintains that the system’s overall structure and approach remain consist...
view detail 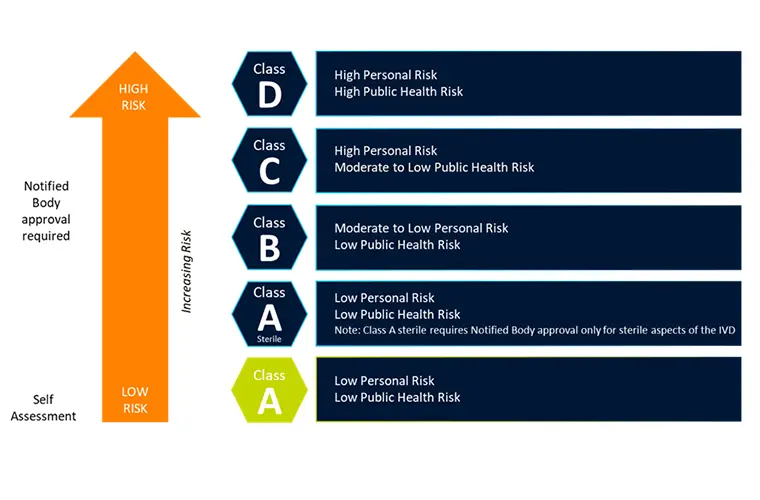
Recent developments in the EU IVD regulatory landscape
2025-03-07
In vitro diagnostics (IVDs) have become a cornerstone of modern medicine, and rising demand for these critical medical devices has driven rapid market growth. Since 2021, hospitals and laboratories...
view detail 
The In Vitro Diagnostics (IVD) market is undergoing significant transformation
2025-03-07
The In Vitro Diagnostics (IVD) market is undergoing significant transformation, driven by technological advancements, shifting market dynamics, and evolving regulatory landscapes. As we navigate th...
view detail 
In Vitro Diagnostic Regulation (IVDR)
2025-03-07
The In Vitro Diagnostic Regulation (IVDR) replaced the IVDD and entered into force on 26 May 2017 with 26 May 2022 as date of applicationIn March 2023, the IVDR was amended as regards to transition...
view detail