+86-18343147735
+86-18343147735 0102030405
News

a2 Milk Company launches new product a2 Pure Health to drive growth in the Chinese market with scientific evidence
2025-07-28
The research and development logic of a2® Zhenzhihu™ is based on a deep insight into the pain points of children's growth. Data shows that after children aged 3-10 enter kindergarten, t...
view detail 
Featured POCT & Diagnostic Reagent Exhibitors
2025-07-01
Here are the standout players in POCT (Point-of-Care Testing) and rapiD Diagnostic reagents showcased at WHX Miami 2025, with a spotlight on innovative immunoassay solutions akin to alternating s...
view detail 
reclassifying hepatitis B and syphilis tests
2025-06-24
《FDA Advances Home‑Use Diagnostic Landscape: Reclassification & Support for POCT》-style report on the FDA’s recent POCT‑focused regulatory shifts — especially reclassifying hepatitis ...
view detail 
WHX Miami 2025 — A Global Hub for Medical Device Innovation & Trade
2025-06-16
Here’s a polished English news-style summary of WHX Miami 2025 (formerly FIME) with key highlights and context: Event Overview WHX Miami 2025, the rebranded Florida International Medical E...
view detail 
FDA Accelerates Home Test Approvals: POCT Is the Future
2025-06-11
1:Policy Shift: Home Diagnostics Enter the Fast Lane
view detail 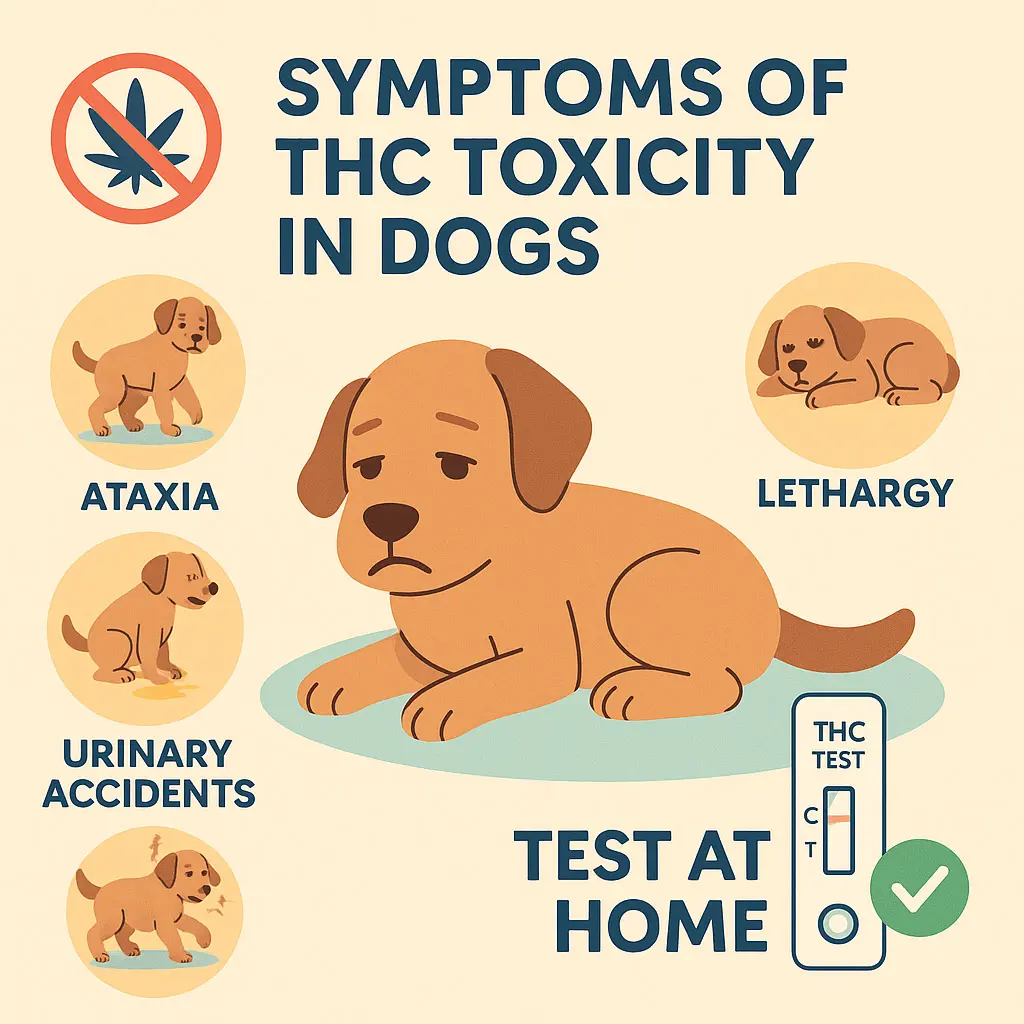
THC in Pets? What Most Owners Don’t Realize
2025-06-05
Unseen Risks, Subtle Symptoms, and the Need for At-Home Testing
view detail 
Understand in 10 Minutes: Home Test vs. Lab Test — What Really Sets Them Apart
2025-06-11
Many assume home testing is “less accurate” or “unprofessional.” In fact, certified home-use kits rely on the same biological principles as lab-based tests—only the sc...
view detail 
FDA-Cleared 12-Panel At-Home Drug Test Kit Empowers Families and Workplaces with Rapid Screening
2025-06-01
Conlight Diagnostics launches an FDA 510(k)-cleared immunoassay Urine cup test, enabling self-administered screening for 12 common drugs of abuse in under 10 minutes. Shanghai, China – June ...
view detail 
The In Vitro Diagnostic Regulation (IVDR) replaced the IVDD and entered into force on 26 May 2017 with 26 May 2022 as date of application
2025-05-16
In March 2023, the IVDR was amended as regards to transitional provisions for certain in vitro diagnostic (IVD) medical devices on Article 110(4) with the removal of the sell-off period to prevent ...
view detail