+86-18343147735
+86-18343147735
- Food Testing
- Pet Testing
- All products
- PCR Kit
- ELISA For Food
- Beta Agonist Rapid Test
- Antibiotic Residue Rapid Test
- Mycotoxin Rapid Test
- Bovine Rapid Test
- Poultry Rapid Test
- Porcine Rapid Test
- Other Rapid Test
- Feline Rapid Test
- Canine Rapid Test
- Sealing Tube Test Strip
- Dog Health Tests
- Product
- Products
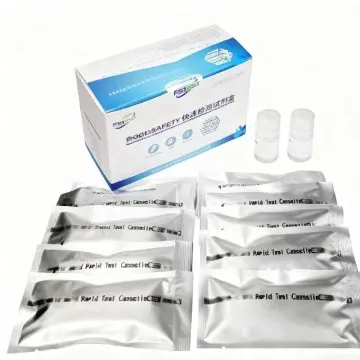
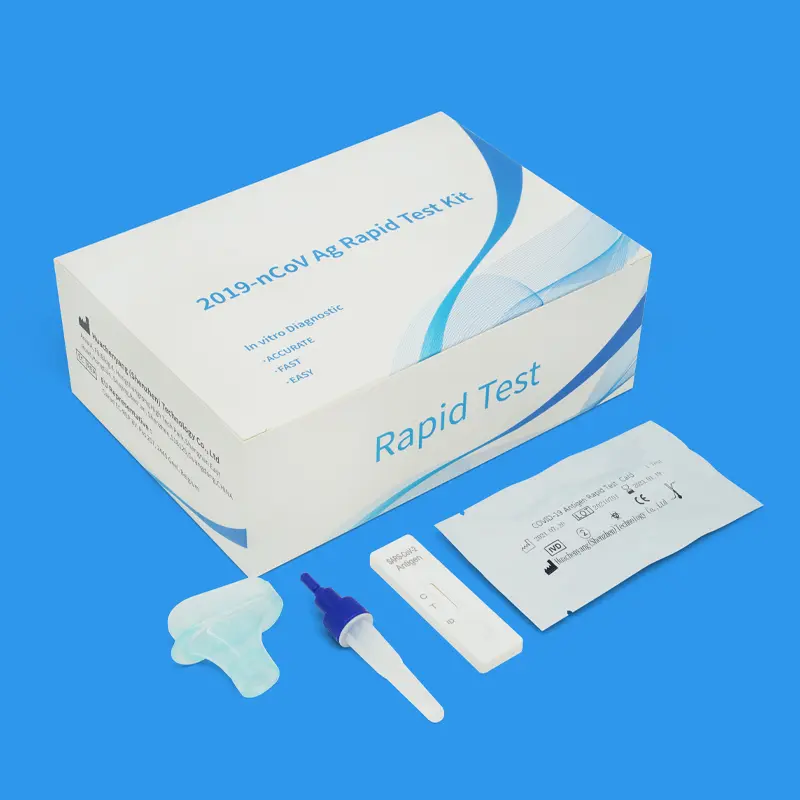
COVID-19 Antigen Rapid Test Kit (25pcs): Saliva Test
product detail
The COVID-19 Antigen Test is an in vitro immunochromatographic method for the qualitative detection of SARS-CoV-2 nucleocapsid protein antigens from the saliva of individuals suspected of COVID-19.
Description of COVID-19 Antigen Rapid Test Kit
The test area (T) on the plain film has been pre-coated with an anti-2019-nCoV monoclonal antibody, forming a red reaction line in the area (T). If the sample does not contain the 2019-nCoV antigen, a red reaction line cannot be formed in the T zone.
A positive test result indicates the presence of viral antigens, but the correlation of clinical, medical history, and other diagnostic information is necessary to determine infection status. However, bacterial infection or co-infection with other viruses can also cause a positive test result.

How to Use 2019-nCoV Ag Rapid Test Kit
| 1 ➜ | 2 ➜ | 3 ➜ | 4 ➜ | 5 ➜ |
|---|---|---|---|---|
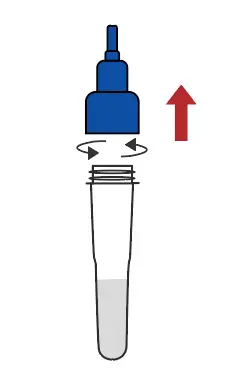 Remove the cap of the extraction buffer |
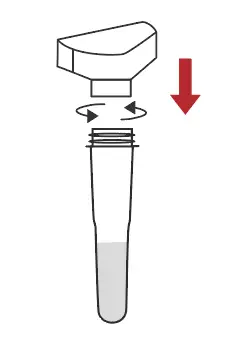 Connect the saliva collector to the extraction buffer tube |
 Cough deeply three times, and spit out saliva from the back of the oropharynx to the collector, saliva volume is up to the line |
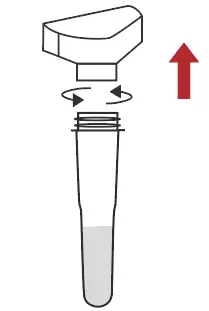 Remove the saliva collector |
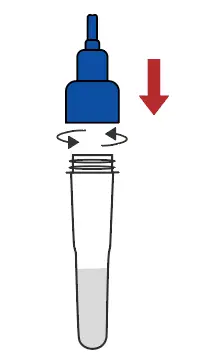 Return the cap |
| 6 ➜ | 7 ➜ | 8 ➜ | 9 ➜ | 10 |
|---|---|---|---|---|
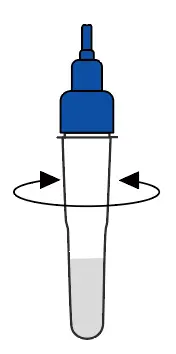 Shake the tube at least 1 min to mix the saliva and extraction buffer thoroughly |
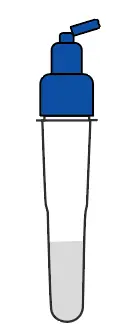 Break the pin on the top |
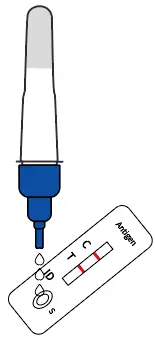 Add 3~4 drops of the processed sample solution (about 80 μL) into the sample well |
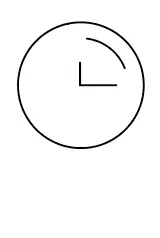 Wait 10-15 minutes |
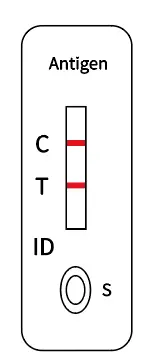 View the results |
NOTE:
- Clean your hands before touching the tool.
- Do not eat for 30 minutes before collection.
- Rinse your mouth with water 30 minutes before saliva collection to clean up the residue.
- Pressing your tongue against the bases of your upper and lower teeth will help you spit out a sufficient amount of saliva in a short amount of time.
- The amount of saliva collected must reach the calibration line.
- Used collection tools should be disposed of in the dedicated clinical waste bin.
Interpretation of the Test Results
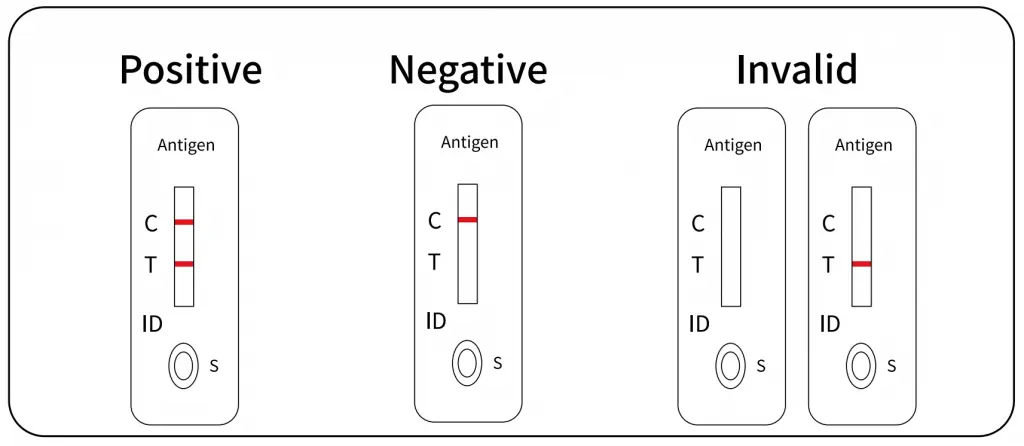
Positive Result:
If both C and T lines are visible within 15 minutes, the test result is positive and valid.
Negative Result:
If the test area (T line) has no color and the control area displays a colored line, the result is negative and valid.
Invalid Result:
The test result is invalid if a colored line does not form in the control region. The sample must be re-tested, using a new test cassette.
NOTE:
- After the test kit is opened, the test should be carried out within 60 minutes.
- Read the result after 15 minutes. The interpretation is invalid for more than 30 minutes.
- When invalid results are presented, you should re-test with a COVID-19 antigen rapid test kit.
Cautions for Using COVID Ag Rapid Test Kit
- Since it cannot be ruled out that the patient is simply infected with the new coronavirus, a negative test result should be considered a presumptive result. The COVID-19 antigen rapid test kit cannot be used as the sole basis for treatment, patient management and infection control. A negative result should be considered based on the patient’s recent exposure, past medical history, and clinical signs and symptoms consistent with COVID-19. If necessary, it should be confirmed by molecular testing in patient management.
- This covid test kit is only used for in vitro auxiliary diagnosis and should be used strictly by the Instructions For Use.
- Please check the expiry date and packaging integrity of the kit before use. If the packaging of the test equipment is damaged or the product is out of date, it cannot be used.
- Due to the limitations of antigen detection kits, it is recommended to use nucleic acid amplification or virus culture identification methods to review and confirm negative test results.
- The kit is stored at 4℃~30℃. Keep away from moist, sunlight, heat, or freezing conditions.
- The test results of this kit are for clinical reference only, and clinical diagnosis should be considered in combination with the patient’s symptoms, signs, medical history, other laboratory tests and treatment response.
- The test results of this COVID-19 antigen rapid test kit are only for clinical reference, and the clinical diagnosis of the disease should be considered in combination with the patient’s symptoms, signs, medical history, other laboratory tests, and treatment response.
“HCY, Health care for you” is our forever mission. We dedicate to offering safe & reliable products and medical services with our global creditable partners. HCY has already supplied to WHO, MAYO clinic, MGI, DDC, Yale University, Qorvo, Quanterix, Thomas Scientific, SD biosensor, Cardinal Health, Cleveland Clinic, Mars Petcare & LumiraDx, etc. in the past years.